Cardiovascular disease (CVD) is humanity’s most lethal disease. According to the " Report on Cardiovascular Health and Diseases in China 2021", CVD patients in our country have reached 330 million, and the number is still rising rapidly with the aging of the population. During the COVID-19 pandemic, CVD patients have been having severer symptoms and a higher mortality rate after infection. Although different classes of anti-CVD drugs have been approved and have made tremendous contributions to cardiac health, they often exhibit side effects on patients due to the complexity of arrhythmia pathogenesis. Therefore, the discovery of new targets for CVD and the development of new anti-CVD drugs are of great significance.

Dr. Hou Panpan (right) and his research group: Postdoctoral Fellow Dr. Yan Zhenzhen (left) and Research Assistant Zhong Ling (middle)
On November 8, 2022, Dr. Hou Panpan, Assistant Professor of the State Key Laboratory of Quality Research in Traditional Chinese Medicine, and his collaborators Professor Guo Jiangtao and Professor Yang Wei from Zhejiang University School of Medicine, published their collaborative research article titled "Structural mechanisms for the activation of human cardiac KCNQ1 channel by electro-mechanical coupling enhancers" in the prestigious journal Proceedings of the National Academy of Sciences (PNAS). Combining cryo-electron microscopy and electrophysiological techniques, this study resolved four high-resolution structures of the human KCNQ1-CaM complex in the apo state (closed), the small molecule agonist ML277 binding state (open), and ML277 with the membrane phospholipid PIP2 binding states (open). These new findings not only elucidate the molecular mechanism of KCNQ1 channel activation by exogenous and endogenous ligands, but also provide promising therapeutics for treating CVD via targeting the KCNQ1 channel.
Dr. Hou has been focusing on the basic and translational research of KCNQ channels for years (Nat. Commun. 2017, 2020; eLife. 2019, 2020). The KCNQ channel family (KCNQ1-KCNQ5) is widely expressed in key organs such as the heart, brain, and digestive system. Accumulating evidence supports that they are potential targets for the treatment of CVD, neuronal disorders, and cancer. For instance, KCNQ1 is abundantly expressed in the heart. Together with its auxiliary subunit KCNE1, the IKs current contributes to the repolarization of cardiac action potentials, and thus plays a crucial role in controlling the heart rhythm. Clinical studies have found that hundreds of loss-of-function mutants of KCNQ1 can cause long QT syndrome (LQT1, accounting for 30-35% of all LQT cases), leading to severe arrhythmias and sudden death. This study provides a new idea for the precise medicine of CVD by targeting the KCNQ1 channel. The study is supported by the Macau Science and Technology Development Fund (FDCT) and the National Natural Science Foundation of China (NSFC).
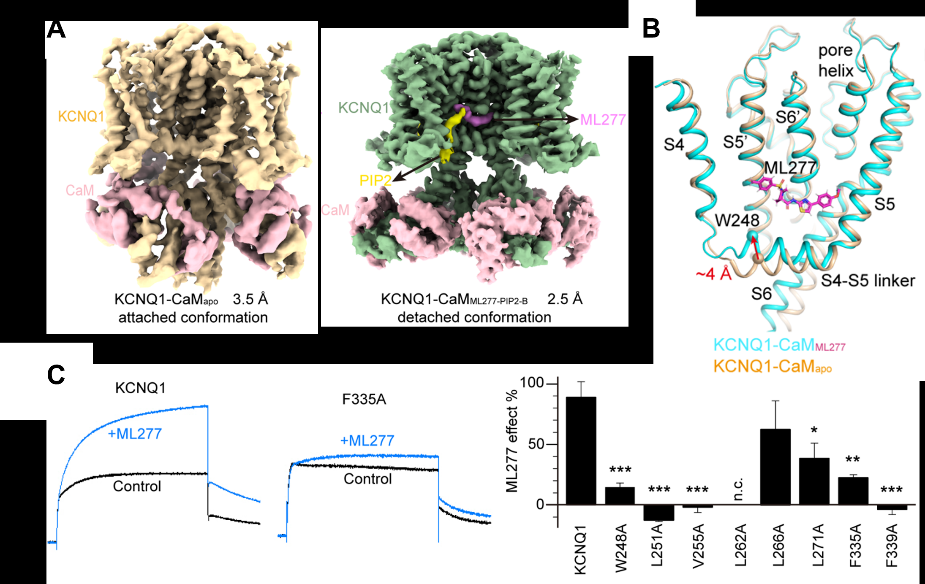
Figure 1. High-resolution structures of KCNQ1 channel with exogenous small molecule ML277 and endogenous phospholipid molecule PIP2. (A) Cryo-EM structures of KCNQ1-CaMApo and KCNQ1-CaMML277-PIP2-B. (B) Structural comparison of KCNQ1-CaMApo and KCNQ1-CaMML277 to show the binding site of ML277 and conformational changes induced by ML277: the N-terminus ("elbow" site) of the S4-S5 linker moves up obviously. (C) Alanine mutation and electrophysiological verification of the ML277 binding site.