M.U.S.T. and Kiang Wu Hospital collaboration: Major research breakthrough in lung cancer treatment by combinational use of Traditional Chinese medicines and monoclonal PD-1 antibody
Sponsored by the Science and Technology Development Fund (FDCT), the research team of Associate Professor Elaine Lai-Han Leung from the State Key Laboratory of Quality Research in Chinese Medicine (Macau University of Science and Technology, M.U.S.T.), and Dr. Yabing Cao from Macau Kiang Wu Hospital, as well as Professor Di Liu from Wuhan Institute of Virology, Professor Hong Wei from the Third Military Medical University, and Professor Jing Yuan from Capital Children’s Research Institute, with Macau University of Science and Technology as the first affiliation, recently published a research article in the well-renowned academic journal Gut (Impact Factor IF: 19.819) entitled “Ginseng polysaccharides alter the gut microbiota and kynurenine/tryptophan ratio, potentiating the anti-tumor effect of anti-programmed cell death 1 /programmed cell death ligand 1 (anti-PD-1/PD-L1) immunotherapy", which is an original discovery of Ginseng anti-cancer basic research worldwide. The research achievement is a successful collaboration between M.U.S.T. and Macau Kiang Wu Hospital, which shows academic progress in personalized and precision treatment of lung cancer. The press conference was held on 11 June 2021 at M.U.S.T. Mr. Wanhei Chan, President of the Administrative Committee of FDCT, Mr. Kunwai Cheang, Member of the Administrative Committee of FDCT, Chair Professor Joseph Hun-wei LEE, Presi-dent of MUST, Chair Professor Liang LIU, Academician of Academy of Chinese Engineering and Director of State Key Laboratory of Quality Research in Chinese Medicine, MUST, Mr. Xuebin Xie, Deputy Director of Kiang Wu Hospital, Dr. He Jing Quan, Chief of Hospital Director’s Office of Ki-ang Wu Hospital, Associate Professor Elaine, Leung Lai Han, Assistant Director of State Key Laboratory of Quality Research in Chinese Medicine, MUST, Dr. Yabing Cao, Director of Depart-ment of Oncology, Kiang Wu Hospital attended the press conference.

Guests at the press conference
Gut is the official journal of the British Society of Gastroenterology and ranks among the top journals in the field of gastroenterology and liver disease. The journal publishes first-class clinical research articles related to the digestive tract, liver, biliary system, and pancreas, with an annual acceptance rate of 9%. Associate Professor Elaine Leung’s research group discovered that ginseng polysaccharides can enhance the response of non-small cell lung cancer (NSCLC) patients to anti-PD-1 immunotherapy by regulating the intestinal flora, which provides a new treatment strategy for the treatment of NSCLC.

Associate Professor Elaine, Leung Lai Han, Assistant Director of QRCMSKL, M.U.S.T. (Left) and Dr. Yabing Cao, Director of Department of Oncology, KWH (Right)
Lung cancer is known as the as the deadliest form of cancer. Although PD-1 immunotherapy has achieved significant and long-lasting effects in some patients, the response rate of PD-1 treatment patients is still low, less than 25%. Therefore, there is an urgent need for more effective first-line treatments for the advanced NSCLC patients. Furthermore, predictive biomarkers are needed to determine which patients may benefit from the new therapies. In this study, the authors used ginseng polysaccharides in combination with PD-1 antibodies and found that the combination therapy can enhance the number of anti-tumor CD8+ immune T cells, resulting in an improvement of the anti-tumor effect of PD-1 immunotherapy, and prolonging the survival time of tumor-bearing mice. Through metabolomics analysis, they found that the level of valeric acid, a metabolite of the flora, was significantly increased, and the ratio of L-kynurenine and L-kynurenine/tryptophan decreased in the mice serum. Using the PacBio third-generation DNA sequencer to analyze the mice feces samples, it was found that the intestinal flora of the mice has changed to a better composition after the combination treatment, which will very much benefit more patients with lung cancer by the combined therapy of PD-1 and product of Ginseng named "Ginsengcare".

Associate Professor Elaine, Leung Lai Han, Assistant Director of QRCMSKL, M.U.S.T
Intestinal flora plays an important role in PD-1 immunotherapy. The author analyzed the stool samples of patients who responded to PD-1 immunotherapy and non-responders from Macau Kiang Wu Hospital using the PacBio third-generation sequencing, and found that the abundance of Bacteroides vulgatus and Parabacteroides distasonis in responders is significantly higher than that in non-responders, which helps to provide accurate information to predict which patients may benefit from the new treatment. In order to further prove that ginseng polysaccharide can enhance the sensitization of PD-1 immunotherapy by regulating the intestinal flora, the stool samples of 6 non-responsive lung cancer patients were transplanted into germ-free mice. After successful colonization, a subcutaneous xenograft tumor model of LLC lung cancer was constructed and mice were co-treated with ginseng polysaccharide and PD-1 treatment again, the results found that the combined treatment can significantly inhibit tumor growth and elevate CD8+ T cells. Intestinal flora analysis found that ginseng polysaccharides can increase the abundance of Bacteroides vulgatus and Parabacteroides distasonis, thereby enhancing the anti-tumor effect of PD-1 antibodies. Metabolomics analysis also found that the level of microbial metabolites valeric acid increased significantly, and the ratio of L-kynurenine and L-kynurenine/tryptophan decreased.
The results of this study indicate that ginseng polysaccharide combined with PD-1 monoclonal antibody is a new strategy to sensitize NSCLC patients to anti-PD-1 immunotherapy. Intestinal microbes can be used as a new biomarker to predict the response of anti-PD-1 immunotherapy. Preclinically, this is also the first report showing that ginseng polysaccharide, a Traditional Chinese Medicine (TCM), can have this new beneficial function. Ginseng is listed as food homology by the National Health Commission of the People's Republic of China, and can be used directly as a food supplement for patients. Therefore, the research team established the industrial production process, carried out small batch industrial production, and transformed the existing findings into a Macau Health Bureau registered product (dietary supplement). The product is named "Ginsengcare" and is undergoing clinically trial. This product can be sold and used as a food supplement product category in Macao, helping to kick-start the translation of R&D of TCM, and promote the combination of basic scientific research and clinical application in Macao.
Gingsengcare product photo
Ph.D. student Jumin Huang and postdoctoral fellow Dr. Yuwei Wang of the State Key Laboratory of Quality Research in Chinese Medicine, Macau University of Science and Technology, and Professor Di Liu from the Wuhan Institute of Virology are the co-first authors of the paper. Dr. Yabing Cao from the Macau Kiang Wu Hospital, Professor Hong Wei from the Third Military Medical University, and Associate Professor Elaine Lai-Han Leung from the State Key Laboratory of Quality Research in Chinese Medicine, Macau University of Science and Technology are the co-corresponding authors of the paper.
The research was funded by the Science and Technology Development Fund of the Macao Special Administrative Region (Modulation of gut microbiota to enhance efficacy of anti-PD1 immunotherapy in non-small cell lung cancer, Project no: 0096/2018/A3 & Dr. Neher’s Biophysics Laboratory for Innovative Drug Discovery, Project no: 001 /2020/ALC).
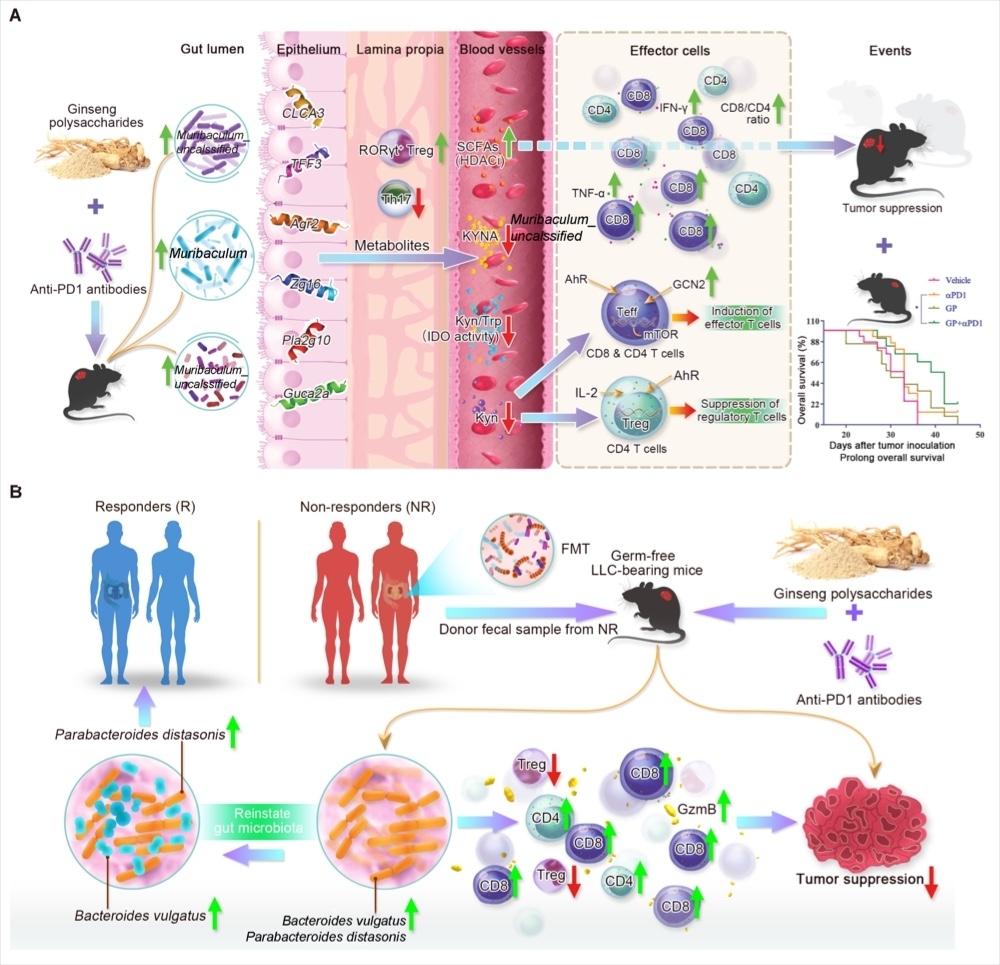
The mechanism of Ginseng Polysaccharides to sensitize anti-PD1 immunotherapy
For viewing the full research paper, please visit the online link from the journal Gut
https://gut.bmj.com/content/early/2021/05/18/gutjnl-2020-321031


